Empowering Sites to Ease the Administrative Burden of Clinical Trials
Scientific innovation in the life sciences industry has accelerated at a rapid pace – the FDA’s Center for Drug Evaluation and Research (CDER) approved 59 novel drugs in 2018, compared with 46 approvals in 2017, and 22 in 2016. While a major victory for patients, this innovation has introduced challenges in clinical trial execution.
With a focus on rare diseases and stratified patient populations, studies have become increasingly complex. Sponsors and CROs have widely adopted technologies to better manage this changing landscape, streamline trial processes, and accelerate end-to-end execution. Now, many clinical research sites are looking to do the same by taking advantage of new cloud-based solutions focused on their needs.
In a recent webinar, “Less Red-Tape, More Research: Reduce the Administrative Burden in Clinical Research,” Megan Blair, the associate director of regulatory affairs at Penn Medicine, discusses their approach for evaluating and adopting new technologies within the organization. She offers tips for sites considering the use of technology to reduce the administrative burden in clinical research.
Conduct an as-is analysis
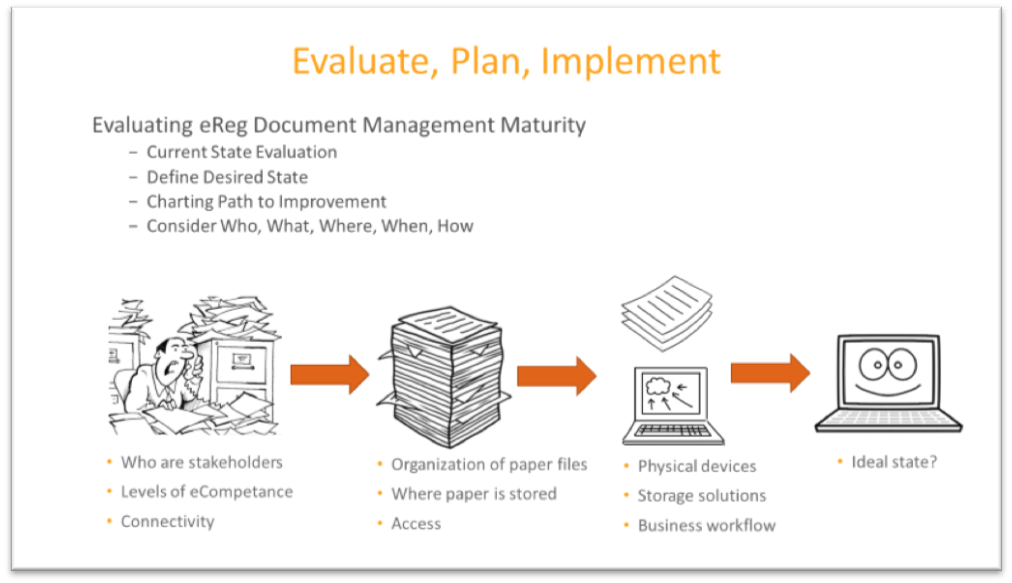
Blair recommends that sites begin with an assessment of their organization’s current processes to uncover areas for greatest improvement. A good starting point is to evaluate site performance against findings from regulatory inspections. At Penn Medicine, this approach resulted in an evaluation of their regulatory document management maturity, with a focus on essential document management, safety reporting, and qualification and training.
The analysis should also include a comprehensive review of the different stakeholders involved, their level of technical competence, the methods used to access and exchange information, and their willingness to adapt to change.
Review the who (stakeholders and affected team members), what (which documents and processes), where (which sites or departments), why (efficiency and/or quality) and how (what tools) questions that will impact the scope of effort. Once these have been documented and understood from all perspectives, sites can define the improved desired state.
Create a strategic plan and roll-out gradually
Rather than try to make a huge change all at once, Blair advises that sites strategically consider which solutions would offer the greatest impact and the order in which to implement.
Penn Medicine began by replacing paper documents with an electronic regulatory binder solution. Once completed, they began to optimize key business processes. For example, they identified key target documents that would benefit most from electronic signatures, ensuring that stakeholder signatories were comfortable with them, and created an SOP for the utilization of electronic signatures.
By starting small and focusing on areas of highest impact, Penn Medicine was able to help their users learn and adapt to changes more easily, while preparing the organization for more complex changes in the future.
Ensure you have appropriate resources
Blair noted that change management initiatives require full-time employee (FTE) investments from the onset. She says it’s important to have people who are capable of not only performing the work, but who also have the time and passion for doing it. She calls this, “financing your future.”
For example, while some of the changes at Penn Medicine required fewer resources to execute, such as adopting electronic signatures on documents and delegation of authority logs, other initiatives were more resource-intensive, such as digitizing physical paper documents. With careful planning, they were able to identify appropriate staffing levels and complete the project on time with quality end-results.
Charting a path to improvement
The introduction of new purpose-built technologies has made it possible for clinical research sites to streamline trial processes and speed study execution. To hear more tips on how your organization can evaluate, plan, and adopt solutions to reduce the administrative burden in clinical research, watch the webinar.
Want exclusive tips and resources?
Be the first to recive upcoming news, join our mailing list.
Subscribe