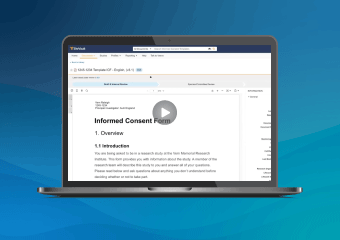
SiteVault eConsent provides a digital way to consent clinical trial participants in-person or remotely.
Break free from legacy tools and cumbersome paper documents. Give your patients access to a modern digital consent experience that fits into their daily lives. Use eConsent across all your studies to drive efficiency, quality, and provide a better patient experience.
SiteVault eConsent is fully validated by Veeva and supports compliance with HIPAA, 21 CFR Part 11, and regional data privacy requirements.
Why Choose SiteVault eConsent
Better Patient Experience
Eliminate the need for patients to carry around paper and provide convenient access to study documents and site information on their own device.
Reduce Staff Burden
Eliminate printing and copying, simplify screening, and easily access documents with a single system that can be used across all studies and works seamlessly with SiteVault.
Stronger Compliance
Reduce errors related to signature dates and times, easily track consent versions, and automatically generate screening logs.
Features
Flexible Consent Options
Enable in-person or remote eConsent on any device. A signed copy of the ICF is stored in the patient's app and can be downloaded anytime.
Compliant eSignatures
Patients and study staff sign ICFs in full compliance with 21 CFR Part 11.
Reporting
Full visibility of patient consent status, date, and version gives sites and monitors the vital information needed to support compliance.
Version and Audit Controls
Automate versioning and view date/time stamps for better compliance and traceability. Easily compare documents to previous versions to see what has changed.
Focused Review
Guide patients with an easy-to-navigate layout and ensure all sections are reviewed prior to sign-off.
Sharing and Collaboration
Easily share and collaborate on informed consent forms between sponsors and sites.
Easily Add Consent Forms
Upload your ICFs and be ready to consent participants in minutes.
Flexible eConsent Templates
Set up your eConsent once and use it for all your study participants. SiteVault eConsent is built to accommodate one or more signers with ease.
Seamless Integration with SiteVault
Manage eConsent across all studies through SiteVault, reducing administrative burden and training requirements.
Validated and Secure
SiteVault eConsent is fully validated by Veeva and supports compliance with HIPAA and regional data privacy requirements.
Frequently Asked Questions
How do I get access to SiteVault eConsent?
SiteVault eConsent can be enabled within your SiteVault account to use across all studies. Contact us to learn more.
Can SiteVault eConsent be used independently from other features in SiteVault?
Yes. While you must have a SiteVault account, you can choose to only use the eConsent functionality within SiteVault on all studies or only specific studies.
Can SiteVault eConsent be used across all my studies?
Yes, creation of eConsent for use across all your studies is available as a paid add-on. SiteVault eConsent is not a sponsor-specific platform, which allows you to standardize your SOPs, workflows, and training for consenting across all studies to improve compliance and efficiency. Learn more about the benefits of a standard eConsent solution here.
Additional Questions?
Get Started Today
Want access to SiteVault eConsent?
Sign Up