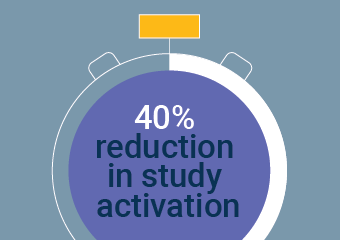
Veeva SiteVault is an eRegulatory application that reduces administrative burden in clinical trials by replacing manual, paper-based processes and connecting you to more than 400 sponsors that use Veeva clinical applications.
Manage regulatory and source documents in a system that supports 21 CFR Part 11 and HIPAA requirements. SiteVault can be used for all studies, regardless of what technology your sponsors use.
See How SiteVault Works
Go Paperless
Eliminate paper, gain visibility into document expirations with intelligent reports and dashboards, and enable remote monitoring.
Enable Remote Monitoring
Eliminate the need to upload and redact documents by providing monitors with secure, direct access to your regulatory and source documents.
Fast Implementation
Get started and turn on remote access for your monitors and your team within 24 hours. There is no download or installation required.
SiteVault Core Features
Full eRegulatory System
Provide investigators and staff with easy access to study documents through an intuitive electronic regulatory binder that supports compliance with 21 CFR Part 11 and HIPAA requirements.
See DemoRemote Monitoring
Provide monitors with secure, direct access to review regulatory and source documents to assist monitors with source data review (SDR) and source data verification (SDV).
See DemoDigital Delegation
Improve the way you manage task delegation with an intelligent, digital process. Gain visibility into staff qualifications and assignments. Process changes more quickly with a delegation log that auto-populates while you work. Ensure compliance with system checks that help identify errors for you.
See DemoeConsent
Manage informed consent forms (ICFs) across all studies through SiteVault, to deliver a better patient experience while creating efficiencies for your site.
Learn MoreAuto-filing and Auto-naming
Reduce manual work and improve compliance with automatic naming and filing of documents. Quickly update CVs, medical licenses, and staff information across multiple studies with a single action.
Connected Studies
Seamlessly exchange documents and study information with sponsors and CROs using Veeva Clinical applications.
Learn MoreVersion Compare
View changes between two versions of a document with version comparison tools. Changes are highlighted eliminating the need for a line-by-line comparison.
Upload Source Documents in Bulk
Save time preparing for monitoring visits by uploading source documents in bulk.
See demoConvert Documents to Searchable Text
Built-in Optical Character Recognition (OCR) converts all files to searchable text, helping you quickly find the documents you need.
Built-in Workflows
Complete document tasks right in the system using eSignatures, certified copy and acknowledgement workflows.
Reports and Dashboards
Improve visibility with powerful reports and dashboards that provide visibility into open tasks, upcoming expiration dates, and signature turn-around timelines.
Secure Cloud Platform
Veeva SiteVault is designed to meet the rigorous content and data management requirements of the life sciences industry.
Learn MoreUnlimited Studies and Users
Both versions support an unlimited number of users and studies and comes with full customer support from Veeva.
25-Year Document Retention Period
Preserve your regulatory documents for up to 25 years in Veeva SiteVault.