Choosing an eISF for Monitoring: How Does SiteVault Compare?
As remote monitoring becomes standard industry practice, clinical research sites must have a compliant solution to support both onsite and remote visits. If your site is choosing an eISF for monitoring — whether it’s sponsor-provided or an eISF that your site will own — consider ways the system will reduce visit prep and training time, make monitoring visits easier, and cut operating costs in the long term.
Here’s How SiteVault Stacks Up
SiteVault
One Place to Manage Documents
When you choose SiteVault — whether it’s because your sponsor offers it or you use it on your own — you will always have one eISF login and one place to track work across all of your studies, regardless of sponsor.
Consistent Ways of Working
Document types, folders, and naming conventions are always consistent, so your team can work efficiently and ensure quality.
Less Training
SiteVault provides a consistent way of working across all studies and sponsors, which means less time training, easier cross-coverage for study teams, and fewer filing errors.
Visit Prep in Minutes
Upload documents in bulk and share central documents (like CVs and CLIAs) across multiple studies without duplication or additional effort. SiteVault automatically compiles documents that are ready for review and tracks monitoring history to save time and avoid re-monitoring.
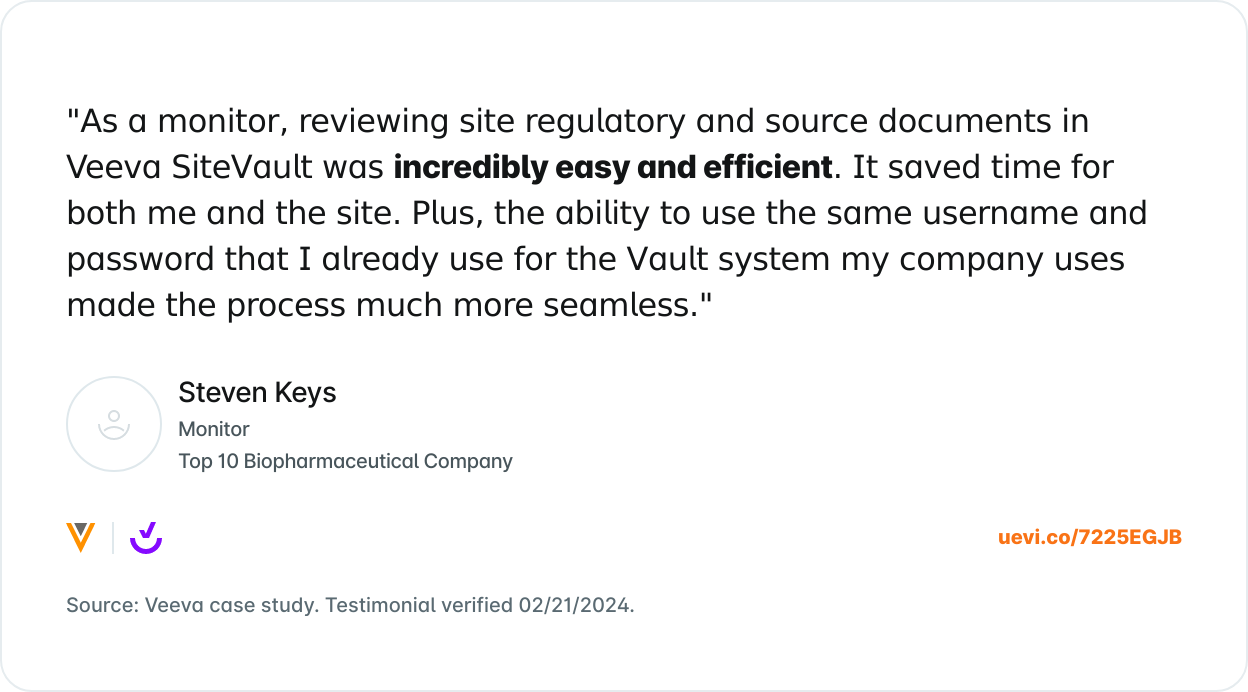
CRAs Can Self-Service
11,000+ CRAs have already used SiteVault, making it the most widely used tool for monitoring. This means less training time and easier, shorter monitoring visits.
Owned and Controlled by You
You have full ownership of your data and documents, can choose which studies to manage in SiteVault, and when to use additional features like eSignatures, Digital Delegation, and document training workflows.
Other eISFs
Multiple Places to Manage Documents
When you work in a sponsor-provided eISF, it typically only works for a single study. Across your team’s studies, this results in multiple eISF logins, disruptions when you transition between studies, and no single place to go to understand the status of document management across your site.
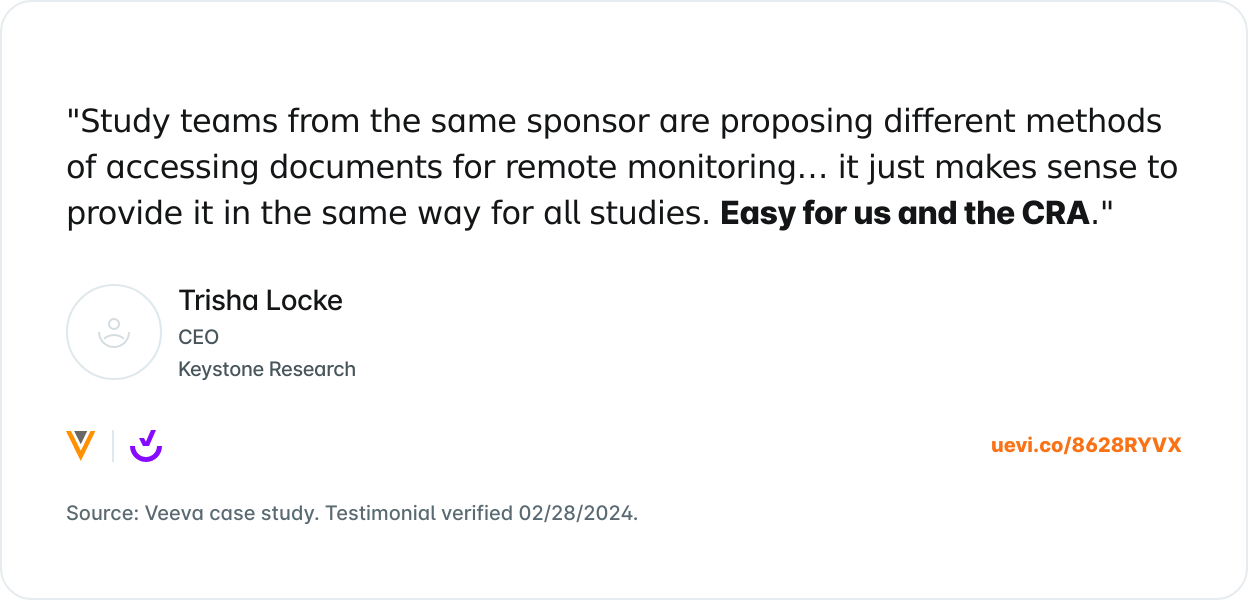
Variability Across Studies
eBinder structures can vary by study or sponsor, which complicates quality oversight and creates extra work to organize, maintain, and find documents.
Training Required for Every Study
Other tools promote too much flexibility in the binder structure, naming conventions, and record-keeping practices — resulting in additional training for each study, wasted time spent remembering where to save documents, and increased risk of misfiling.
Hands-On Visit Prep
Manually file and tag documents to studies one-by-one, and compile the documents for monitoring by hand, resulting in extra work and increased risk of monitoring errors.
CRAs Need Oversight
You’ll teach your CRA how to use your eISF, show them where documents are filed, and manually check to make sure they only access what’s appropriate.
Owned and Controlled by Your Sponsor
If your eISF is provided by your sponsor, they control how you work, including which studies you can manage in the system, and which features you can use.
Starting is Quick and Easy
Sign up to unlock access in just 1-2 days. Then follow our straightforward, step-by-step guide to prepare for your first monitoring visit.
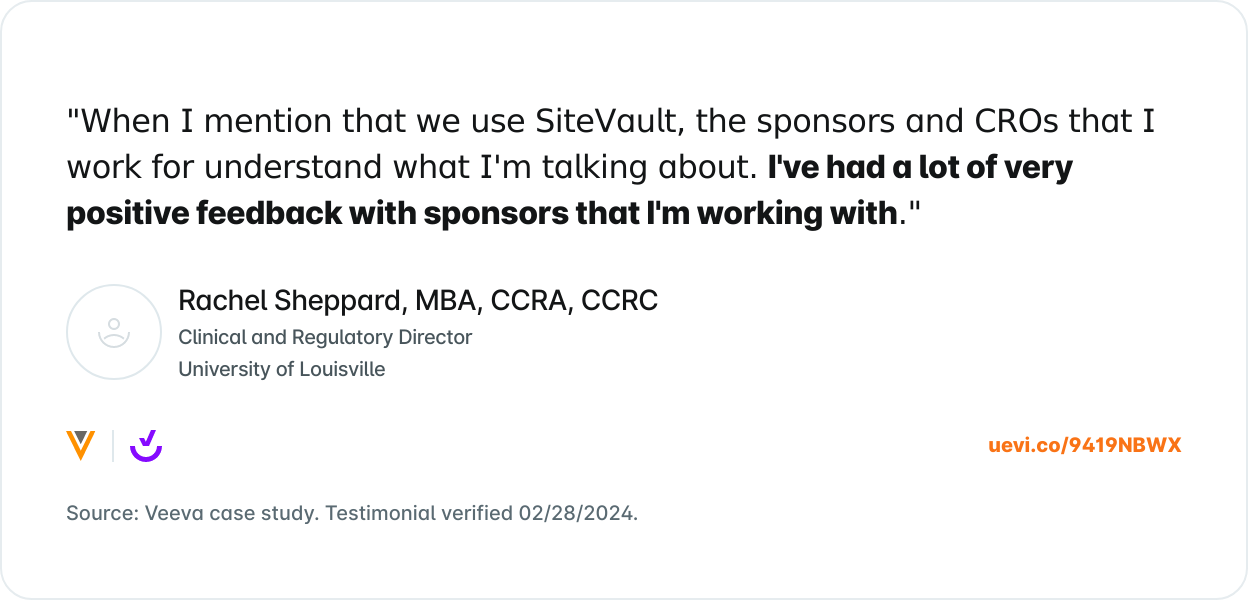
Sponsor & CRO Approved
Veeva is a household name among sponsors and CROs, with 18 of the top 20 biopharmas and 4 of the top 6 CROs already using our technology to run their trials.
24/7 Live Support
Includes on-demand chat and phone support in all time zones and most languages. You’ll also get access to self-service guides and videos to learn at your own pace.
See How it Works
SiteVault makes it easy to prepare for visits and empower monitors to self-service.
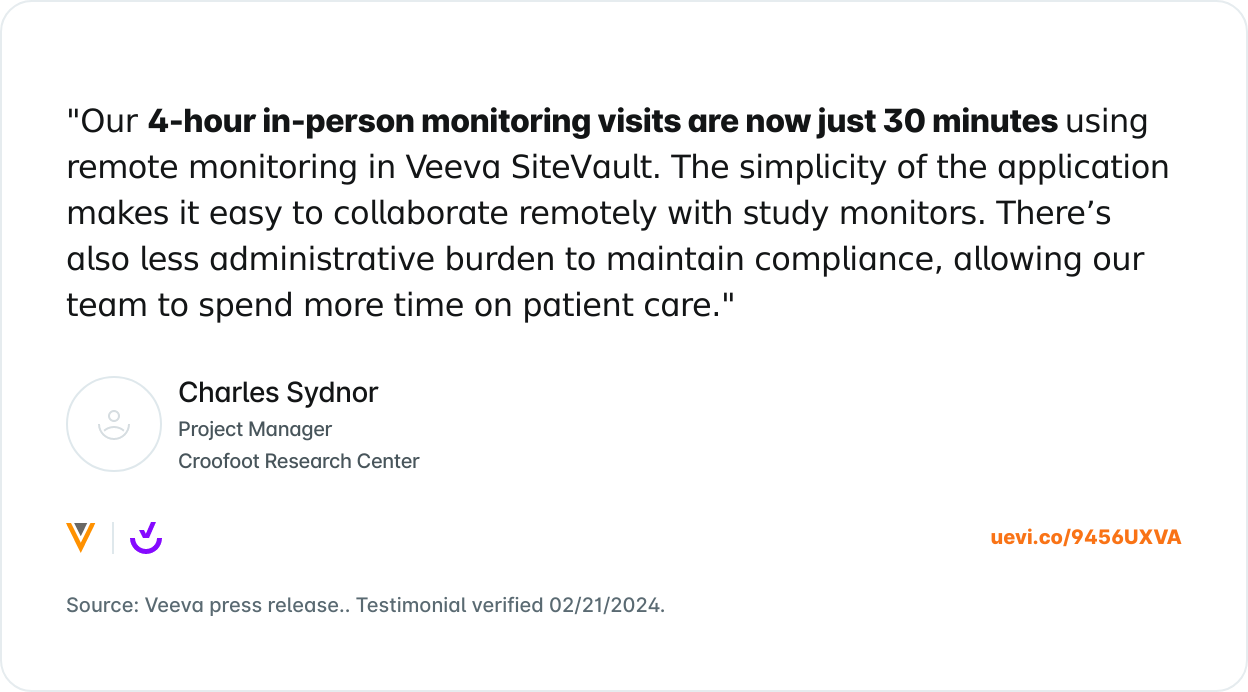
What Others are Saying
Trusted by 8,000+ sites and counting.
Join the growing number of research sites that are using SiteVault for remote monitoring.
Sign up today, it's free!