Veeva Announces Site Connect to Automate Information Sharing in Clinical Trials
New application will connect sponsors and sites for better collaboration and faster study execution
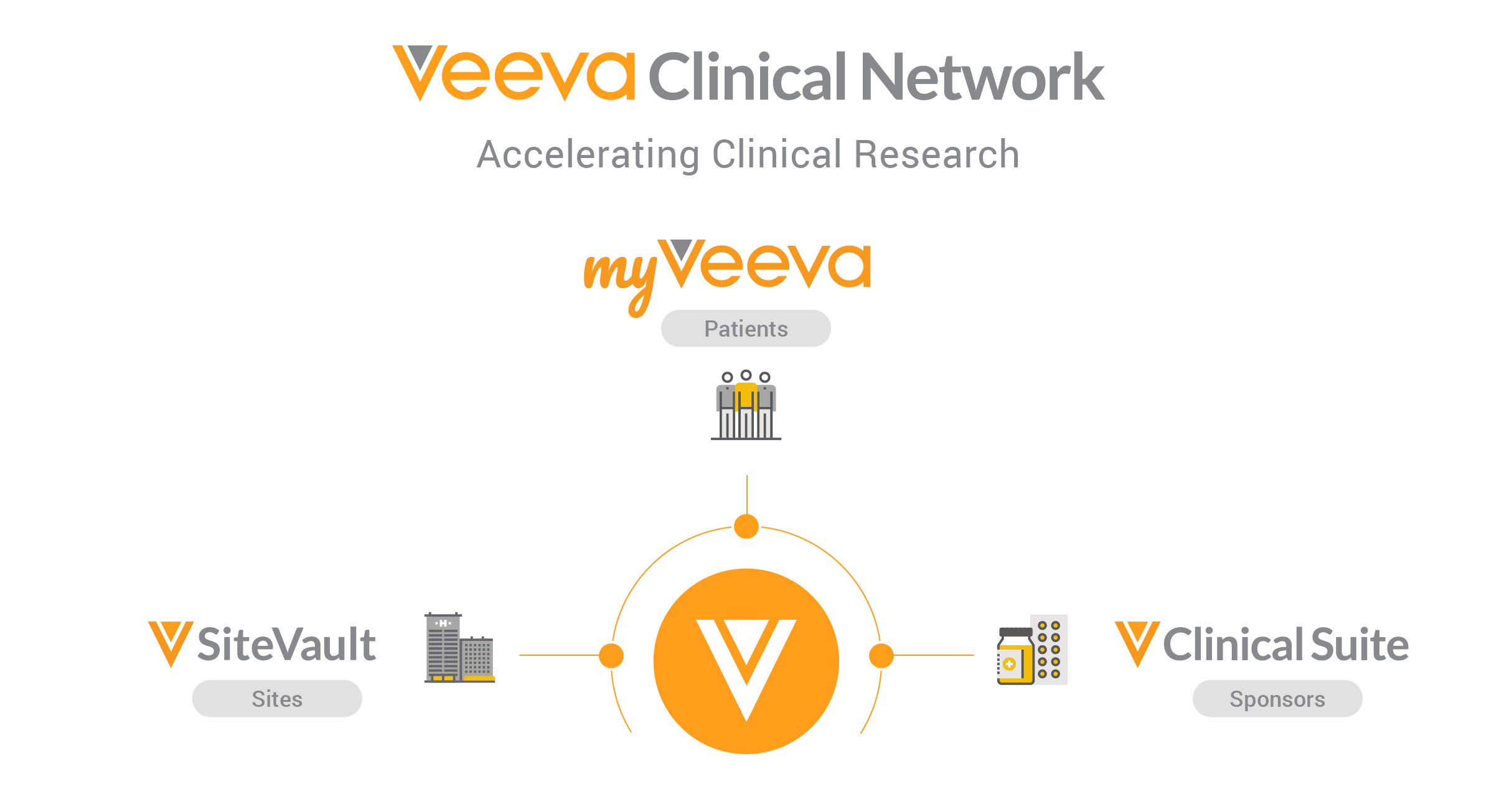
Veeva Clinical Network will bring together sponsors, sites, and now patients to accelerate clinical research
PLEASANTON, CA — May 19, 2020 — Veeva Systems (NYSE:VEEV) today announced Veeva Site Connect, a new application that connects sponsors and clinical research sites during trials. Veeva Site Connect automates the flow of information between Vault Clinical applications used by sponsors and Veeva SiteVault, a compliant eISF application used by sites for source document management and remote monitoring. Now sponsors and sites can streamline information sharing for key trial processes, including feasibility, study document exchange, safety letter distribution, and subject status for faster study execution.
“There is a significant opportunity to speed information exchange throughout the clinical trial process,” said Doug Schantz, member of SCRS leadership council and former head of U.S. site management and monitoring at AstraZeneca. “Veeva Site Connect will transform how sponsors and sites work together and help get studies up and running much faster.”
Veeva Site Connect seamlessly automates information flow across trial partners, processes, and systems. Sponsors can easily collect input from sites on study feasibility to spend less time coordinating surveys; accelerate document exchange and reconciliation between eTMF and eISF; quickly distribute safety letters to notify global sites and regulators of an adverse event; and get real-time information on the status of patients from enrollment through treatment – all with one application.
With Veeva Site Connect, sponsors can improve site engagement by reducing the time and effort in exchanging clinical information. Now sites can focus less on administrative tasks and responding to information requests and more on execution and treating patients, improving overall study quality.
“Veeva Site Connect will eliminate the many portal interfaces, manual handoffs, and multiple steps it takes to share data with sponsors during a trial,” said Tonya Yarbrough, director, Vanderbilt Institute for Clinical and Translational Research at Vanderbilt University Medical Center. “Now the industry can leverage the same innovative Veeva Vault technology to drive greater efficiency and productivity during trials.”
Veeva Site Connect is part of the Veeva Clinical Network, a set of solutions that brings together sponsors, sites, and patients to accelerate clinical research. In addition to Site Connect, Veeva today announced MyVeeva, a multichannel patient portal for virtual visits and patient-centric trials. Together with Veeva Vault Clinical Suite, Veeva is the first and only company providing solutions that help sponsors, sites, and patients share and view information during a clinical trial.
“Veeva aims to improve efficiency and collaboration across the clinical trial ecosystem,” said Jim Reilly, vice president of Veeva Vault R&D. “With applications for sponsors, sites, and patients all part of the Veeva Clinical Network, we are providing a common way to connect and get real-time information throughout the course of a study.”
Veeva Site Connect is planned for availability in August 2020. To learn how Site Connect automates information sharing in clinical trials, watch this on-demand webinar.
Additional Information
For more on Veeva Site Connect, visit: veeva.com/SiteConnect
Connect with Veeva on LinkedIn: linkedin.com/company/veeva-systems
Follow @veevasystems on Twitter: twitter.com/veevasystems
Like Veeva on Facebook: facebook.com/veevasystems
About Veeva Systems
Veeva Systems Inc. is the leader in cloud-based software for the global life sciences industry. Committed to innovation, product excellence, and customer success, Veeva serves more than 850 customers, ranging from the world’s largest pharmaceutical companies to emerging biotechs. Veeva is headquartered in the San Francisco Bay Area, with offices throughout North America, Europe, Asia, and Latin America. For more information, visit veeva.com.
Forward-looking Statements
This release contains forward-looking statements, including the market demand for and acceptance of Veeva’s products and services, the results from use of Veeva’s products and services, and general business conditions, particularly in the life sciences industry. Any forward-looking statements contained in this press release are based upon Veeva’s historical performance and its current plans, estimates, and expectations, and are not a representation that such plans, estimates, or expectations will be achieved. These forward-looking statements represent Veeva’s expectations as of the date of this press announcement. Subsequent events may cause these expectations to change, and Veeva disclaims any obligation to update the forward-looking statements in the future. These forward-looking statements are subject to known and unknown risks and uncertainties that may cause actual results to differ materially. Additional risks and uncertainties that could affect Veeva’s financial results are included under the captions, “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” in the company’s filing on Form 10-K for the period ended January 31, 2020. This is available on the company’s website at veeva.com under the Investors section and on the SEC’s website at sec.gov. Further information on potential risks that could affect actual results will be included in other filings Veeva makes with the SEC from time to time.
###
Contact:
Roger Villareal
Veeva Systems
925-264-8885
roger.villareal@veeva.com
Deivis Mercado
Veeva Systems
925-226-8821
deivis.mercado@veeva.com
Want exclusive tips and resources?
Be the first to recive upcoming news, join our mailing list.
Subscribe