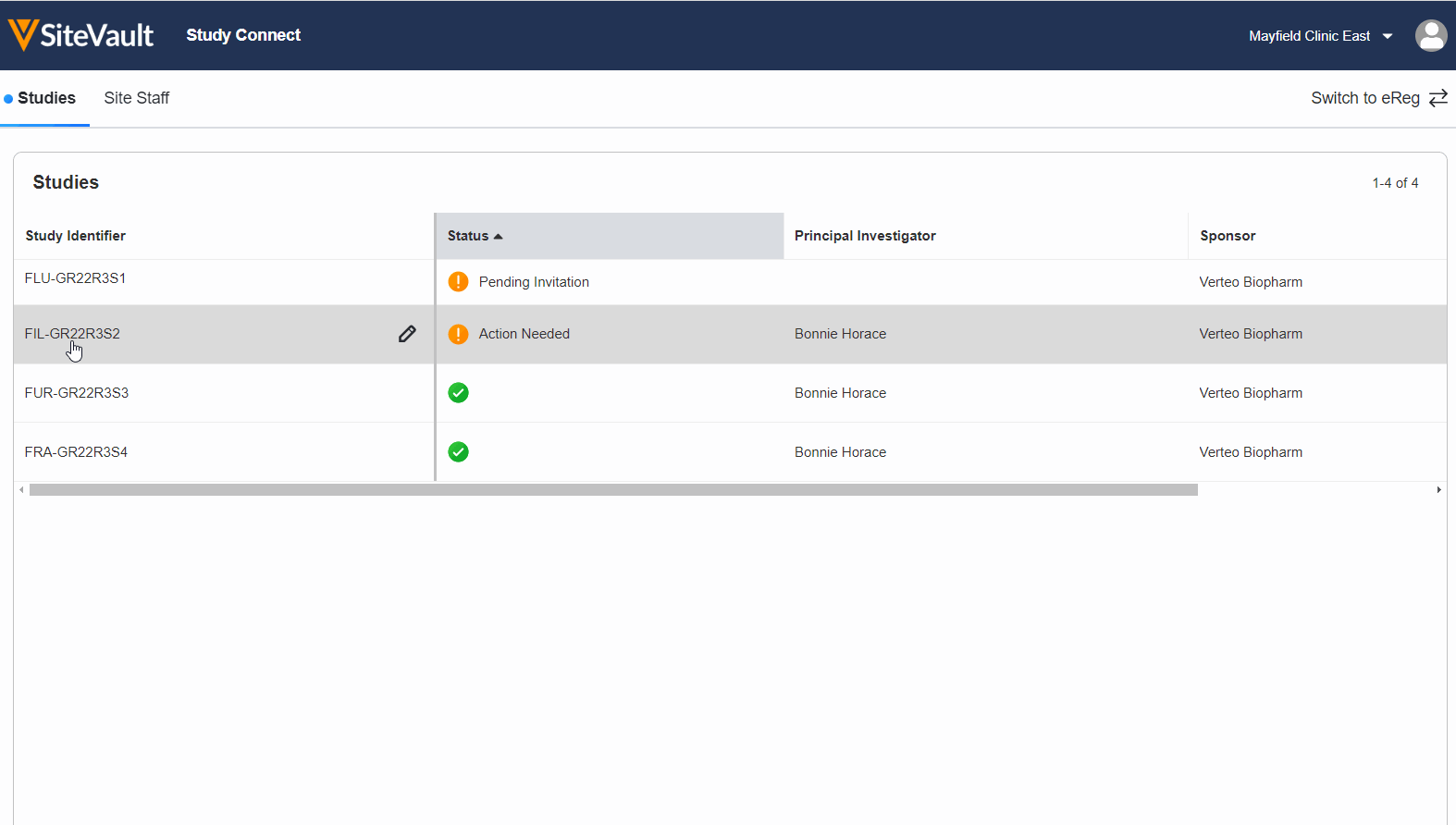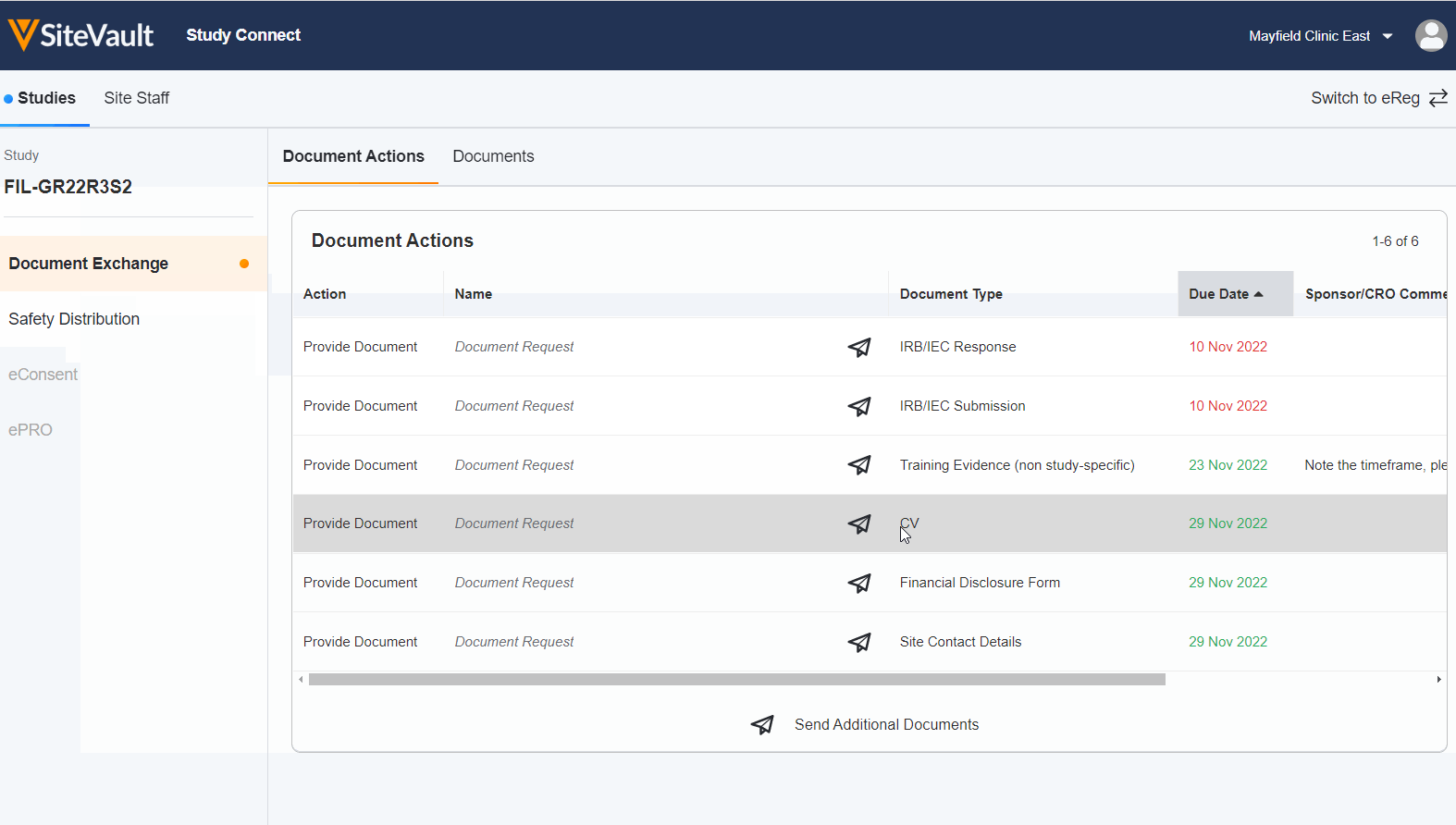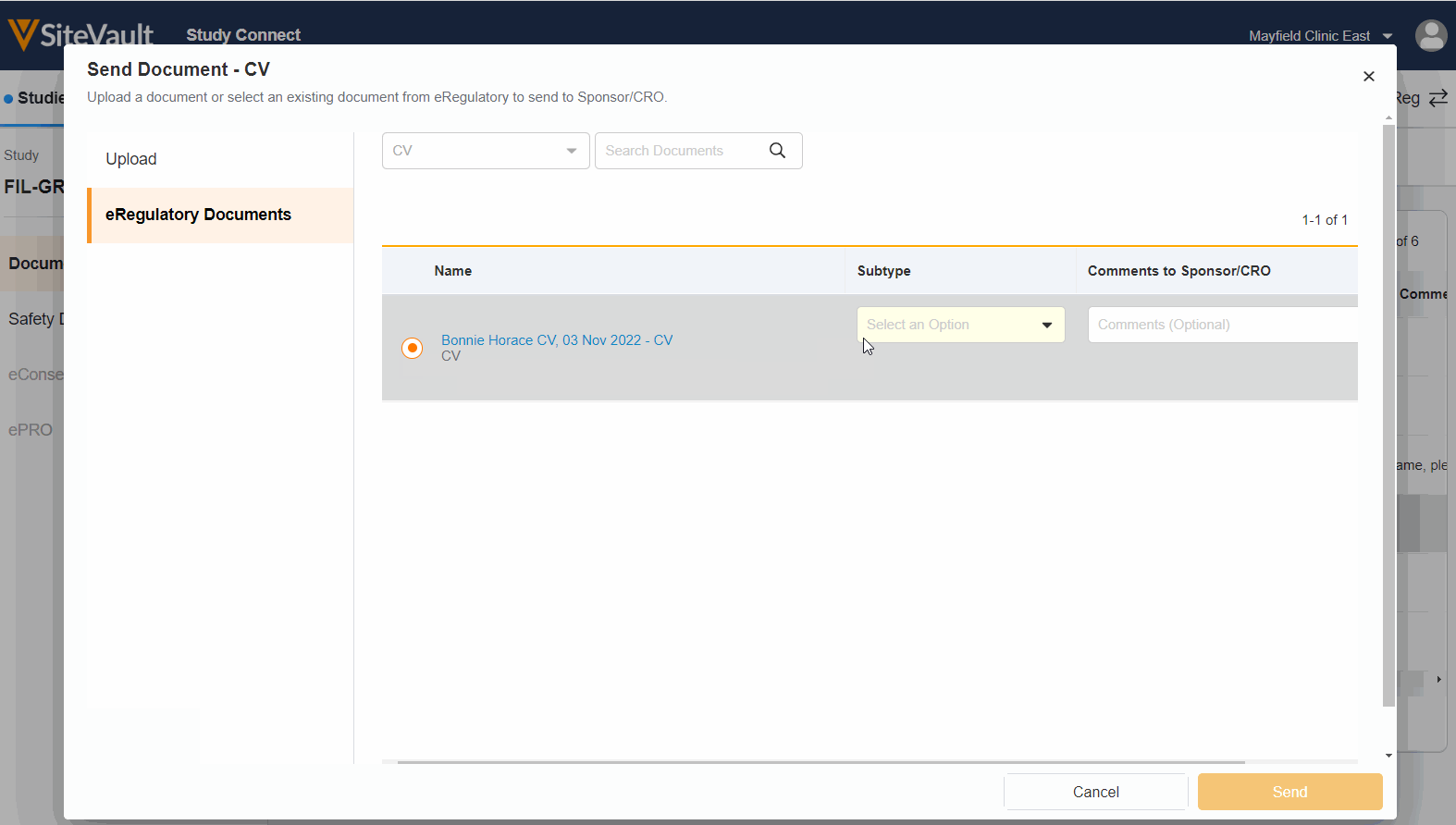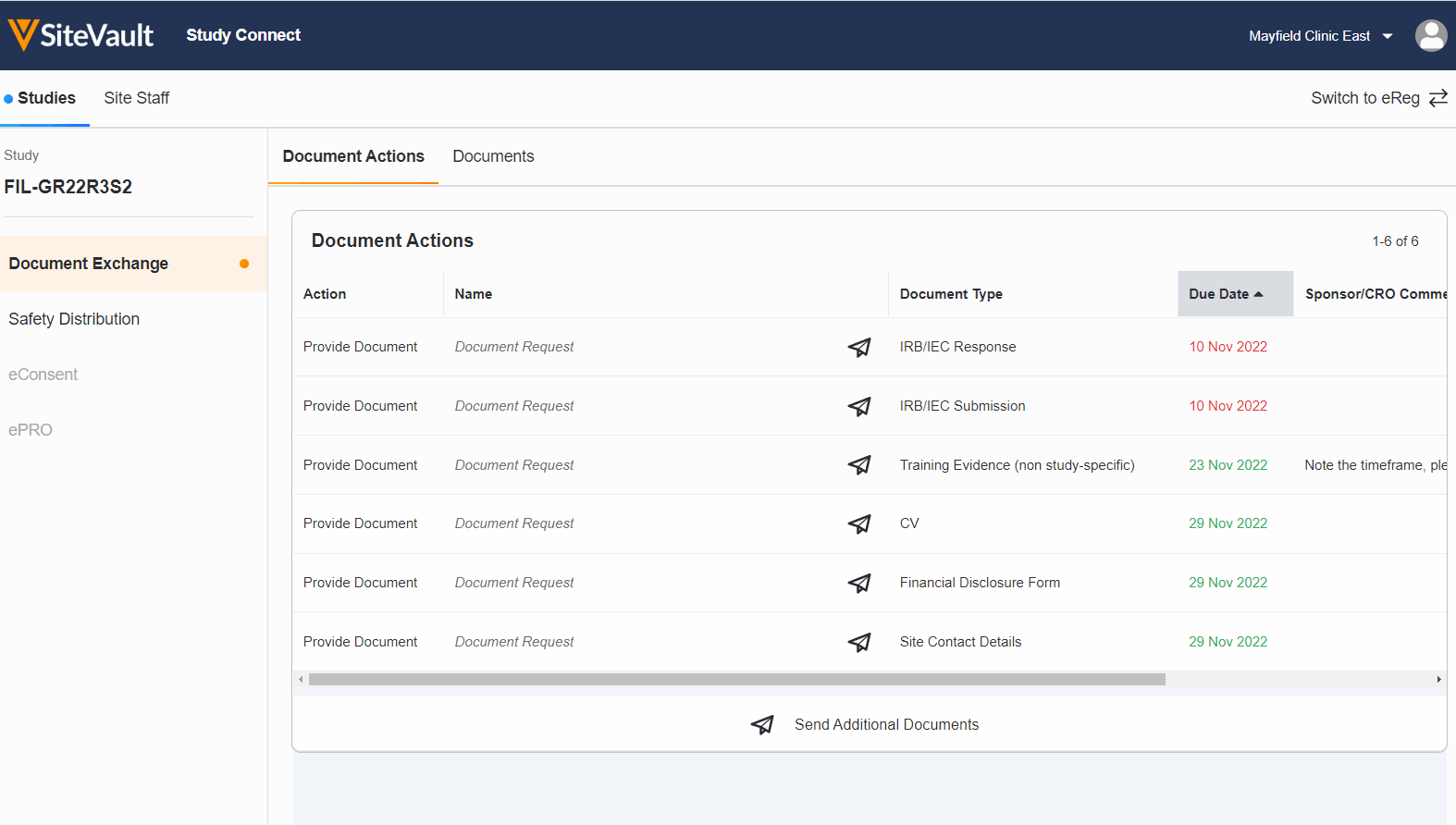
The current technology landscape is not sustainable for research sites, who are inundated with a growing number of systems to learn and use on top of their already heavy workload.
Study Connect saves time and reduces technology burden by providing a single place for sites to exchange trial information with sponsors. Sites can easily send documents, receive and acknowledge safety reports, and capture patient eConsent and ePRO in a single system.
Unlike traditional sponsor-provided tools, setup is easy, there’s no training required, and the site maintains a historical view of everything exchanged with sponsors.
Study Connect integrates seamlessly with SiteVault eReg, and is built on the same platform that 400+ sponsors and CROs already use to run their trials. Signup today.
Information Sharing Made Easy
Easily exchange study information with less effort through a single application that’s seamlessly connected to sponsors and CROs without third-party integrations.
Speed Study Start-up and Execution
Complete study start-up activities, share trial information, and communicate with your sponsor or CRO in one place to save time and reduce repetitive work.
Choice and Control
Collaborate without compromising data privacy, security, and control. Retain full visibility of historical data after study close-out in a secure, site-owned system.
Capabilities
Seamless Document Exchange
Receive and exchange start-up packages, regulatory documents, invoices, payment info, and end-of-study media to reduce manual and redundant work.
Receive and Acknowledge Safety Letters
Simplify the receipt and acknowledgment process around safety letters to save time and ensure investigators stay informed.
Auto-file Documents into eReg
Provide investigators and staff with quick and easy access to information with a system that automatically files study documents into SiteVault eReg.
Learn more about eRegManage eConsent
Make patient participation in clinical trials easier by providing convenient access to consent forms and key study information.
Learn moreManage ePRO
Simplify the setup, collection, and review of ePRO and provide patients with a single, easy-to-use application for all their trial activities.
Frequently Asked Questions
How do I get started with Study Connect?
Sites will be invited to share documents within Veeva SiteVault by the trial sponsor or CRO.
How can I get help setting up Study Connect?
Veeva provides comprehensive support to help sites set up Study Connect including self-help resources, live and on-demand training, phone/chat/email support, and a dedicated site success team.
Can Study Connect be used independently from other features in SiteVault?
Yes. While you must have a Veeva SiteVault account, you can choose to only use the Study Connect functionality where requested by your sponsor.